Silver is extracted from the anode waste sludges of electrolytic copper-refining. Isotopes: Silver has 35 isotopes whose half-lives are known, with mass numbers 94 to 128. Naturally occurring silver is a mixture of its two stable isotopes, 107 Ag and 109 Ag with natural abundances of 51.8% and 48.2% respectively. Mass number and isotopic mass. The mass number gives an estimate of the isotopic mass measured in atomic mass units (u). For 12 C, the isotopic mass is exactly 12, since the atomic mass unit is defined as 1/12 of the mass of 12 C. For other isotopes, the isotopic mass is usually within 0.1 u of the mass number. For example, 35 Cl (17 protons.
Mass Numbers - How to find Mass Numbers
The mass number is established by rounding the atomic weight to the nearest whole number. The Periodic Table with Atomic Masswill give you the atomic weight, or atomic mass, of the elements. The chemical properties of an element are determined by its Atomic Number not its Mass Number which is why atomic numbers are shown on the Periodic table whilst Mass Numbers are not. Mass numbers equal the total number of heavy, or massive, particles in the nucleus. Subtracting the Atomic number from the Mass Number equals the number of neutrons in the nucleus.
Mass Numbers = Atomic Weight of Element, rounded to nearest whole number
Number of Neutrons = Mass Number - Atomic Number
Mass Numbers
Mass Numbers - The Mass Numbers of all of the elements
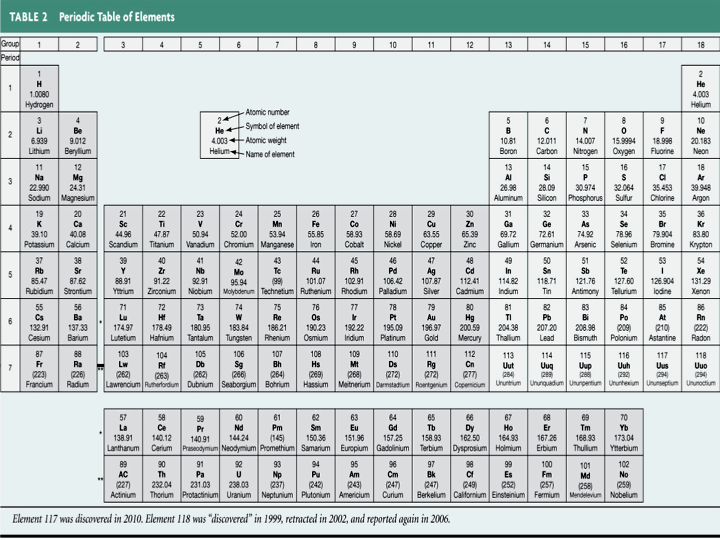
So, if we know the number of protons and neutrons in an atom we can determine the mass number. The unique chart below has been created by www.elementalmatter.info and details all of the elements in the Periodic table, the numbers of protons, the numbers of neutrons and the mass numbers of atoms which relate to the elements.
Mass numbers - Examples of Mass Numbers
The following examples provide details of how to calculate the mass number.
- Example 1 - mass number of Gold: The element Gold (Symbol Au) has the Atomic Number of 79. The number of protons in atom of gold is therefore 79. Gold has the Atomic Mass weight of 196.97. Round to the nearest whole number. The mass number of gold is therefore 197.
- Example 2 - mass number of Silver: The element Silver (Symbol Ag) has the Atomic Number of 47. The number of protons in atom of silver is therefore 47. Silver has the Atomic Mass weight of 107.87. Round to the nearest whole number. The mass number of silver is therefore 108.
- Example 3 - mass number of Neon: The element Neon (Symbol Ne) has the Atomic Number of 10. The number of protons in atom of neon is therefore 10. Neon has the Atomic Mass weight of 20.18. Round to the nearest whole number. The mass number of neon is therefore 20.
Mass Numbers = Atomic Weight of Element, rounded to nearest whole number
Mass numbers - Chart of Mass Numbers
The details all of the elements in the Periodic table, the numbers of protons, the numbers of neutrons and the mass numbers of atoms which relate to the elements in the Periodic Table.
Chart of Mass Numbers

| Atomic Number | Name of Element | Symbol of Element | Mass Numbers | Name of Element |
| 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 | Hydrogen Helium Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon Sodium Magnesium Aluminium Silicon Phosphorus Sulfur Chlorine Argon Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium Germanium Arsenic Selenium Bromine Krypton Rubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium Ruthenium Rhodium Palladium Silver Cadmium Indium Tin Antimony Tellurium Iodine Xenon Cesium Barium Lanthanum Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Holmium Erbium Thulium Ytterbium Lutetium Hafnium Tantalum Tungsten Rhenium Osmium Iridium Platinum Gold Mercury Thallium Lead Bismuth Polonium Astatine Radon Francium Radium Actinium Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Californium Einsteinium Fermium Mendelevium Nobelium Lawrencium Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Ununbium Ununtrium Ununquadium Ununpentium Ununhexium Ununseptium Ununoctium | H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Rf Db Sg Bh Hs Mt Ds Rg Uub Uut Uuq Uup Uuh Uus Uuo | 1 4 7 9 11 12 14 16 19 20 23 24 27 28 31 32 35 40 40 40 45 48 51 52 55 56 58 58 64 65 70 73 75 79 80 84 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 133 137 139 140 141 144 145 150 152 157 159 163 165 167 169 173 175 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 223 226 227 232 231 238 237 244 243 247 247 251 252 257 258 259 262 261 268 263 264 269 268 272 273 277 286 289 288 292 292 293 | Hydrogen Helium Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon Sodium Magnesium Aluminium Silicon Phosphorus Sulfur Chlorine Argon Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium Germanium Arsenic Selenium Bromine Krypton Rubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium Ruthenium Rhodium Palladium Silver Cadmium Indium Tin Antimony Tellurium Iodine Xenon Cesium Barium Lanthanum Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Holmium Erbium Thulium Ytterbium Lutetium Hafnium Tantalum Tungsten Rhenium Osmium Iridium Platinum Gold Mercury Thallium Lead Bismuth Polonium Astatine Radon Francium Radium Actinium Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Californium Einsteinium Fermium Mendelevium Nobelium Lawrencium Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Ununbium Ununtrium Ununquadium Ununpentium Ununhexium Ununseptium Ununoctium |
| Atomic Number | Name of Element | Symbol of Element | Mass Numbers | Name of Element |
Chart of Mass Numbers
Element Silver - Ag
Comprehensive data on the chemical element Silver is provided on this page; including scores of properties, element names in many languages, most known nuclides of Silver. Common chemical compounds are also provided for many elements. In addition technical terms are linked to their definitions and the menu contains links to related articles that are a great aid in one's studies.
Silver Menu

- Silver Page One
- Silver Page Two
- Silver Page Three
How Many Protons Are In Silver
Overview of Silver
- Atomic Number: 47
- Group: 11
- Period: 5
- Series: Transition Metals
Silver Mass Number And Atomic Number
Silver's Name in Other Languages
- Latin: Argentum
- Czech: Stříbro
- Croatian: Srebro
- French: Argent
- German: Silber - s
- Italian: Argento
- Norwegian: Sølv
- Portuguese: Prata
- Russian: Серебро
- Spanish: Plata
- Swedish: Silver
Atomic Structure of Silver
- Atomic Radius: 1.75Å
- Atomic Volume: 10.3cm3/mol
- Covalent Radius: 1.34Å
- Cross Section (Thermal Neutron Capture)σa/barns: 63.6
- Crystal Structure: Cubic face centered
- Electron Configuration:
- 1s2 2s2p6 3s2p6d10 4s2p6d10 5s1
- Electrons per Energy Level: 2,8,18,18,1
- Shell Model
- Shell Model
- Ionic Radius: 1.26Å
- Filling Orbital: 4d10
- Number of Electrons (with no charge): 47
- Number of Neutrons (most common/stable nuclide): 61
- Number of Protons: 47
- Oxidation States: 1
- Valence Electrons: 4d10 5s1
Chemical Properties of Silver
- Electrochemical Equivalent: 4.0246g/amp-hr
- Electron Work Function: 4.26eV
- Electronegativity: 1.93 (Pauling); 1.42 (Allrod Rochow)
- Heat of Fusion: 11.3kJ/mol
- Incompatibilities:
- Acetylene, ammonia, hydrogen peroxide, bromoazide, chlorine trifluoride, ethyleneimine, oxalic acid, tartaric acid
- Ionization Potential
- First: 7.576
- Second: 21.49
- Third: 34.83
- Valence Electron Potential (-eV): 11.4
Physical Properties of Silver
- Atomic Mass Average: 107.8682
- Boiling Point: 2436K 2163°C 3925°F
- Coefficient of lineal thermal expansion/K-1: 19.2E-6
- Conductivity
- Electrical: 0.63 106/cm Ω
Thermal: 4.29 W/cmK
- Electrical: 0.63 106/cm Ω
- Density: 10.5g/cc @ 300K
- Description:
- Very soft and malleable silver metal. Appearance and odor vary depending upon specific compound.
- Elastic Modulus:
- Bulk: 103.6/GPa
- Rigidity: 30.3/GPa
- Youngs: 82.7/GPa
- Enthalpy of Atomization: 284.5 kJ/mole @ 25°C
- Enthalpy of Fusion: 11.3 kJ/mole
- Enthalpy of Vaporization: 255.1 kJ/mole
- Flammablity Class: Non-combustible solid (except as dust)
- Freezing Point:see melting point
- Hardness Scale
- Brinell: 24.5 MN m-2
- Mohs: 2.5
- Vickers: 251 MN m-2
- Heat of Vaporization: 250.58kJ/mol
- Melting Point: 1234K 961°C 1762°F
- Molar Volume: 10.27 cm3/mole
- Optical Reflectivity: 97%
- Physical State (at 20°C & 1atm): Solid
- Specific Heat: 0.235J/gK
- Vapor Pressure = 0.342Pa@961°C
Regulatory / Health
- CAS Number
- 7440-22-4
- RTECS: VW3500000
- NFPA 704
- Health: 1
- Fire: 2
- Reactivity:
- Special Hazard:
- OSHAPermissible Exposure Limit (PEL)
- TWA: 0.01 mg/m3
- OSHA PEL Vacated 1989
- TWA: 0.01 mg/m3
- NIOSHRecommended Exposure Limit (REL)
- TWA: 0.01 mg/m3
- IDLH: 10 mg/m3
- Routes of Exposure: Inhalation; Ingestion; Skin and/or eye contact
- Target Organs: Nasal septum, skin, eyes
- Levels In Humans:
Note: this data represents naturally occuring levels of elements in the typical human, it DOES NOT represent recommended daily allowances.- Blood/mg dm-3: <0.003
- Bone/p.p.m: 0.01-0.44
- Liver/p.p.m: 0.005-0.25
- Muscle/p.p.m: 0.009-0.28
- Daily Dietary Intake: 0.0014-0.08 mg
- Total Mass In Avg. 70kg human: 2 mg
Who / Where / When / How
- Discoverer: Known to ancient civilization
- Discovery Location: Unknown
- Discovery Year: Unknown
- Name Origin:
- Latin argentum (silver). Silver from Anglo-Saxon seolfor for silver.
- Abundance of Silver:
- Earth's Crust/p.p.m.: 0.07
- Seawater/p.p.m.:
- Atlantic Suface: N/A
- Atlantic Deep: N/A
- Pacific Surface: 0.0000001
- Pacific Deep: 0.0000024
- Atmosphere/p.p.m.: N/A
- Sun (Relative to H=1E12): 7.1
- Sources of Silver:
- Found in ores called argentite (AgS), light ruby silver (Ag3 AsS3), dark ruby silver (Ag3SbS3) and brittle silver. Silver is often obtained as a by-product of refining other metals like copper and gold. World wide production is around 9950 tons per year. Primary mining areas are Mexico, Bolivia, Honduras, Canada, USA.
- Uses of Silver:
- Used in alloys for jewelry, in many compounds, photographic film and paper electronics, mirrors and batteries.
- Additional Notes:
Silver Menu
- Silver Page One
- Silver Page Two
- Silver Page Three
References
Twixtor pro for mac. A list of reference sources used to compile the data provided on our periodic table of elements can be found on the main periodic table page.
Related Resources
- Anatomy of the Atom
Answers many questions regarding the structure of atoms. - Molarity, Molality and Normality
Introduces stoichiometry and explains the differences between molarity, molality and normality. - Molar Mass Calculations and Javascript Calculator
Molar mass calculations are explained and there is a JavaScript calculator to aid calculations. - Chemical Database
This database focuses on the most common chemical compounds used in the home and industry.
Silver Periodic Table Mass Number
Citing this page
If you need to cite this page, you can copy this text:
Kenneth Barbalace. Periodic Table of Elements - Silver - Ag. EnvironmentalChemistry.com. 1995 - 2021. Accessed on-line: 4/24/2021
https://EnvironmentalChemistry.com/yogi/periodic/Ag.html
.
Linking to this page
If you would like to link to this page from your website, blog, etc., copy and paste this link code (in red) and modify it to suit your needs:
<a href='https://EnvironmentalChemistry.com/yogi/periodic/Ag.html'>echo Periodic Table of Elements: Silver - Ag (EnvironmentalChemistry.com)</a>- Comprehensive information for the element Silver - Ag is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions.
.
NOTICE: While linking to articles is encouraged, OUR ARTICLES MAY NOT BE COPIED TO OR REPUBLISHED ON ANOTHER WEBSITE UNDER ANY CIRCUMSTANCES.
PLEASE, if you like an article we published simply link to it on our website do not republish it.
